Oxidative stress and chronic inflammation are two phenomena that are directly involved in various diseases, from metabolic to neurological disorders and even in the formation of tumor [1]. In this context, a wide source of bioactive molecules exhibiting anti-inflammatory effects, combined with antioxidant potential, can be found in natural extracts [2]. Whereas previously, as a source of these phytochemicals, the focus was on the 'nobler' parts of the plant, i.e. the fruits, leaves and roots, today much emphasis is placed on investigating by-products from the agribusiness production chain. Specifically, this strategy aimed at the reuse of industrial by-products could reduce the impact of cultivation on waste production, minimizing disposal problems, while obtaining a potential source of bioactive compounds.
In particular, it has recently been reported that certain phytochemicals are able to target two closely related systems within the cell, namely the NRF2 and NF-kb pathways [3], [4]. While the NRF2 pathway is responsible for regulating an extensive panel of antioxidant enzymes, NF-kb is the main effector pathway involved in inflammation. Phytochemicals in general mediate their effects by modulating the DNA-binding capacity of both these transcriptional factors and, in turn, correcting the state of cellular imbalance related to an uncontrolled inflammatory condition. In particular, apple-specific polyphenols exert antioxidant, anti-inflammatory and lipid-lowering effects due to the components contained in every part of the fruit (peel, stark, seeds and pulp). This biological activity was also confirmed in preclinical studies in which isolated apple polyphenols (AP) were shown to be effective in the prevention/treatment of various metabolic and inflammatory diseases, from to hypercholesterolemia [5] to ulcerative colitis [6].
Since apples are one of the most consumed fruits in the world, the related waste generated at all stages of the agricultural process has a great impact on the environment. The thinning process in particular, i.e. the removal and dumping of young apples one month after blossom to guarantee the quality of the harvested ripe apples, heavily contributes on the generation of waste of potentially bioactive material. Young apples, in fact, are particularly rich in polyphenols (almost 10-fold compared to harvested ripe apples), and could be exploited as such [7].
During the first two years of my PhD, I mainly focused on the qualitative profiling of a thinned apple polyphenol extract (TAP) using LC-HRMS (positive and negative ionic mode) approaches, followed by the exploration of its potential anti-inflammatory and antioxidant activity using multiple cell-based assays and the latest proteomics strategies; main results were published in a peer-reviewed article in 2022 [8]. Figure 1 illustrates and resumes the preject design.
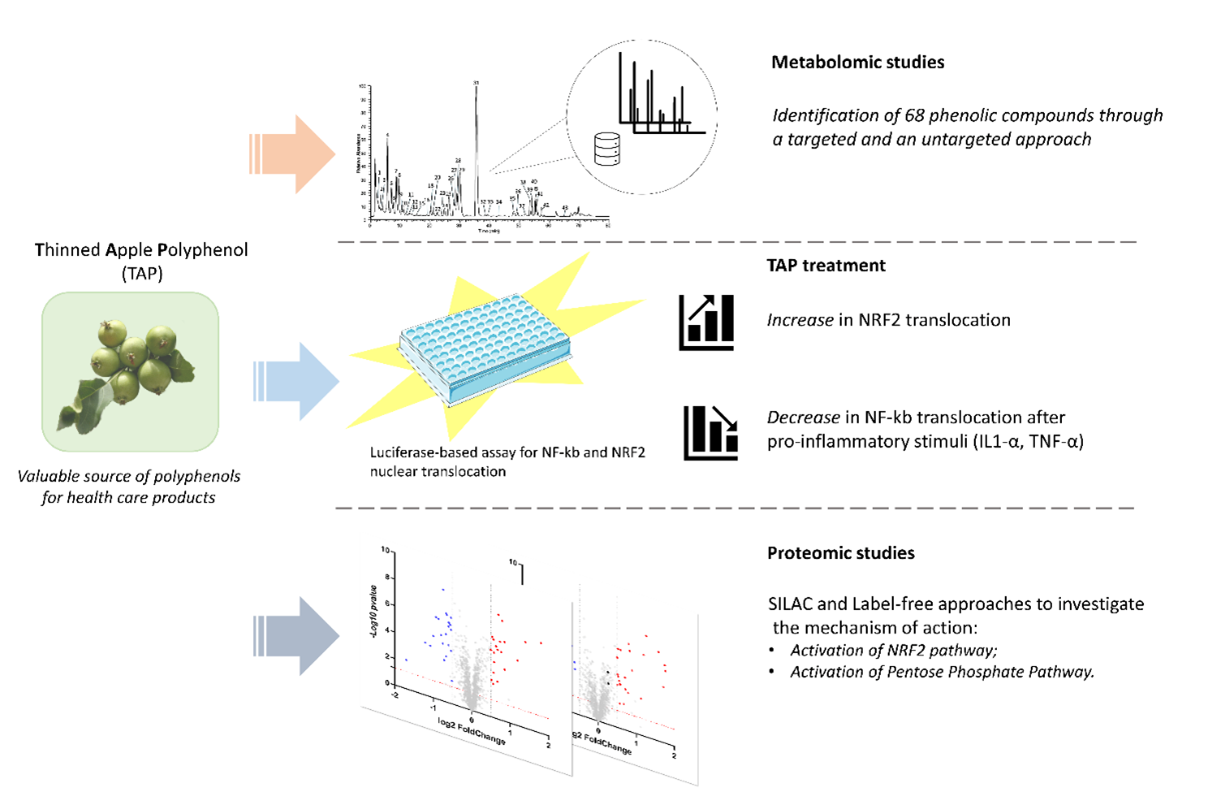
Figure 1: Graphical representation of the different approaches used to define the qualitative profile and to evaluate the biological activity of thinned apple polyphenols (TAP) extract.
A targeted approach was used to identify 52 compounds using an in-house library of potential apple metabolites, while 16 were identified by an untargeted approach based on computing the elemental composition of each newfound ion, used to search an online database that generates probable candidates from the chemical formula; in turn, each candidate has to be validated and, only if certain, identified as being present in the extract. Of a total of 68 compounds, 65 were polyphenols; 39 compounds commonly detected in both polarities, while 7 were unique in negative ion mode, and 6 in positive. In detail, among the 52 compounds identified by the targeted approach, 20 were phenolic and organic acids, 11 flavanols, 10 flavonols, 5 flavanones, 4 dihydrochalchones, 1 flavone (luteolin) and 1 triterpenoid (euscaphic acid). The 16 compounds identified using the untargeted approach are instead classified as follows: 9 flavonols, 3 phenolic acids, 1 flavanone, 1 dihydrochalchone and 2 lipids.
Once the concentration of total polyphenols in the TAP extract had been determined, the radical scavenging activity of the extract was assessed using the DPPH assay, revealing an activity significantly higher than the reference compounds when expressed on the basis of polyphenol content. The radical scavenging activity determined suggests a potential direct antioxidant activity of the extract, although the most accepted hypothesis to date refers to an indirect antioxidant defense mechanism involving the activation of the NRF2 pathway; moieties such as ortho-diphenols, which characterize some polyphenols, once oxidized to the corresponding quinones in an environment characterized by a marked oxidative stress, will acquire a more electrophilic character that will allow these molecules to interact with the highly reactive cysteines of the Kelch-like ECH-associated protein 1 (KEAP1); the latter interacts with NRF2 in a redox-sensitive manner and the dissociation of the proteins in the cytoplasm is followed by transportation of NRF2 to the nucleus.
This hypothesis was confirmed by treating a NRF2/ARE Responsive Luciferase Reporter HEK293 stable cell line treated with increasing concentrations of TAP extract (1 - 250 μg/mL) for 6 or 18 hours. After 6 hours, the effect started to be significant at a concentration of 50 μg/mL to induce a 2.3-fold increase at a 250 μg/mL concentration. The fold increase was higher after an incubation time of 18 hours to reach more than a 5-fold increase at the highest tested dose. In parallel, the extract (TAP) was also tested on a NF-kb Responsive Luciferase Reporter R3/1 stable cell line challenged with two different pro-inflammatory agents: TNF- α and Il-1 α for 6 hours or 24 hours, respectively. NF-kB induced luciferase was dose-dependently reduced by TAP pre-treatment (16 hours).
In order to acquire information concerning the impact of the phytocomplex on the proteome of a cellular model of inflammation, a quantitative proteomic study was conducted using both a SILAC (Stable Isotope Labelling by Amino acids in Cell culture) and a LFQ (label-free quantitative) approaches. The experiment was designed to evaluate the effect of TAP on the cellular proteome under homeostatic conditions and thus to exclude any toxicity effect, and to test its efficacy under a pro-inflammatory stimulus-induced oxidative stress condition. Although the two approaches are characterized by different levels of performance (e.g., different number of proteins identified and significantly quantified), the same modulated cellular pathways were identified, making the results more robust.
As expected, many up-regulated genes involved in the NRF2 pathway activation were detected. Besides, the crucial finding is that TAP treatment over-expresses the inducible isoform of heme oxygenase (HMOX1), a well-established immunomodulator [10]; the induction of HMOX1 protects against the cytotoxicity caused by oxidative stress and apoptotic cell death, making HMOX1 an appealing target for the treatment of several chronic inflammatory diseases [11], [12].
In conclusion, these first results, suggested that thinned apples can be effectively considered a valuable source of apple polyphenols to be used in health care products to prevent/treat oxidative and inflammatory chronic conditions.
To critically evaluate if the extract could efficiently dampen an inflammatory condition in vivo, the extract was orally administered (10 mg/kg) in a murine DNBS model of ulcerative colitis (UC). The incidence rate of UC, one of the Inflammatory Bowel Diseases (IBDs), has been increased in the last decades [13]. Although the etiology is still unclear, the most accepted hypothesis centers on defects in tight junctions that result in increased intestinal permeability, leading to increased entry of luminal antigens that contribute to intestinal inflammation. Currently available drug therapies represent symptomatic treatment, with the goal of keeping patients in a state of remission to avoid flare-ups, which greatly affect his or her quality of life. Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids succeed in partially relieving the symptoms of the disease and, to date, are considered the drugs of choice for the treatment of ulcerative colitis, although they are associated with non-negligible side effects. Hence the interest in finding new therapeutic strategies that counteract inflammation as effectively without inducing damage in other body districts. Since oxidative stress and inflammation contribute to tissue damage during colitis, the administration of natural compounds with antioxidant and anti-inflammatory activity has recently been proposed as a treatment for IBD. Apple polyphenols for instance are effective compounds in inhibiting microbial dysbiosis, chronic inflammation, and modulating intestinal permeability, and could therefore be used as dietary supplements to improve gut health. Given the characteristics of thinned apple by-product and with a view to sustainability, we moved to evaluate the therapeutic potential of TAP extract.
This project was carried out during my third year of PhD in collaboration with the University of Messina which handled the treatment of the animals (mice) and collected the colon tissue after sacrifice.
Using state of the art techniques in the field of proteomics we performed ex vivo functional studies to describe, at a molecular level, the biological processes distinctive for the pathological phenotype, and to understand the molecular pathways evoked by TAP extract to effectively limit the inflammatory state. In detail, more than 5400 proteins were identified and quantified in colon tissues from to different experimental groups, according to the canonical label-free quantitative (LFQ) proteomic approach as previously described [8]. These data were then interpreted and rationalized using gene ontology annotation-based software, such as STRING and Ingenuity Pathway Analysis.
Overall, proteomics studies confirmed the usefulness of the chosen animal model characterized by immune cells infiltration and marked oxidative stress, and outlined the following molecular pathways evoked by TAP treatment: (i) activation of antioxidant-acting mechanisms; (ii) reversal of mechanisms overexpressed/activated in the presence of DNBS, with particular reference to mechanisms of ferroptosis and heme-toxicity; (iii) inhibition of the immune response; (iv) down-regulation of agents implicated in wound healing, as a reduced ulcerative condition may have generated less tissue damage and, consequently, less need for activation of coagulation, inflammation and angiogenesis processes. A summary of these results is reported in Figure 2 below.
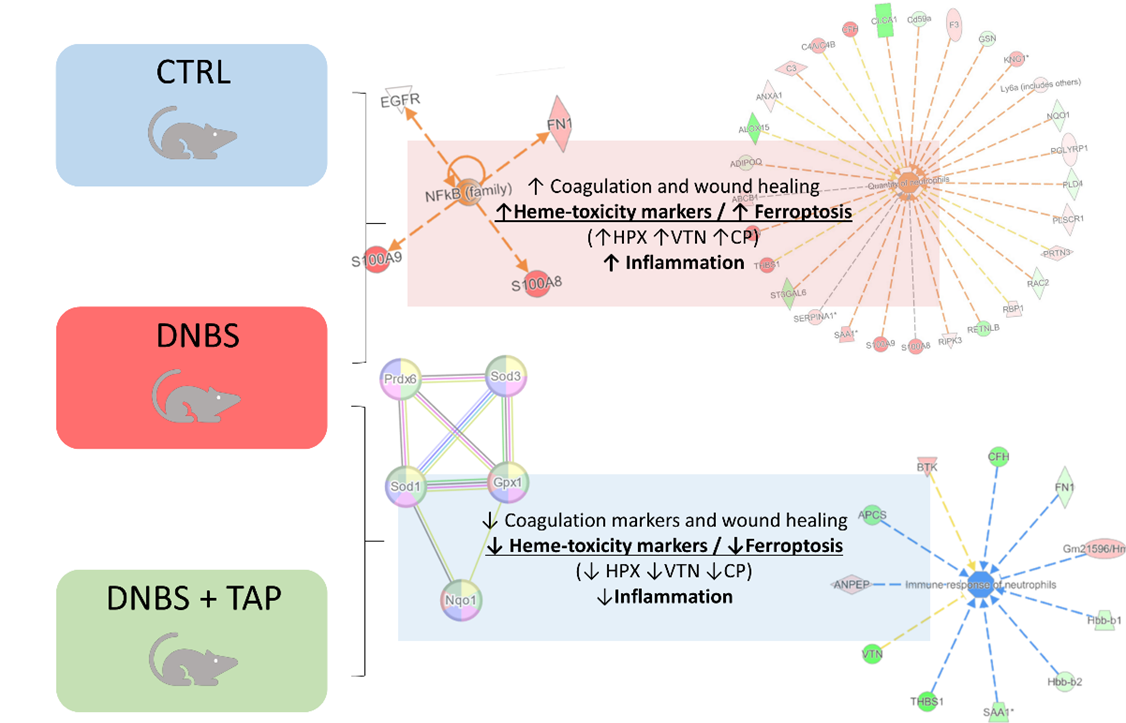
Figure 2: Graphical representation of the main results from the quantitative proteomic study, from top to bottom the hallmarks of the DNBS-induced disease state and the effect of TAP extract reverting this condition.
Taken together, these results suggest that thinned apples may be considered a valuable source of polyphenols for use in health products to prevent/treat chronic inflammatory conditions; nevertheless, thinned apples represent a novel and as yet unexplored waste product for industrial production of bioactive extracts with significant economic and environmental impacts.
References
[1] Pisoschi A. M., Pop A. Eur. J. Med. Chem. 2015, 97: 55-74
[2] Zhu F., Du B., Xu B., Crit. Rev. Food Sci. Nutr., 2018, 58: 1260-1270
[3] Qin S., Hou D.-X., Mol. Nutr. Food Res., 2016, 60: 1731-1755
[4] Tanigawa S., Fujii M., Hou D., Free Radic. Biol. Med., 2007, 42: 1690-1703
[5] Aprikian O.., Busserolles J., Manach C., Mazur A., Morand C., Davicco M.J., Besson C., Rayssiguier Y., Remesy C., Demigne C., J. Nutr., 2002, 132: 1969-1976
[6] Yeganeh P. R., Leahy J., Spahis S., Patey N., Desjardins Y., Roy D., and Delvin E., Garofalo C., Leduc-Gaudet, J., St-Pierre D., Beaulieu J., Marette A., Gouspillou G., Levy E., J. Nutr. Biochem., 2018, 57: 56-66
[7] Dou J., Meng Y., Liu L., Li J., Ren D., Guo Y., Int. J. Biol. Macromol., 2015, 72: 31-40
[8] Ferrario G., Baron G., Gado F., Della Vedova L., Bombardelli E., Carini M., D'Amato A., Aldini G., Altomare A., Antioxidants, 2022, 11: 1577
[9] Sun L., Guo Y., Fu C., Li J., Li Z., Food Chem., 2013, 136: 1022-1029
[10] Campbell N. K., Fitzgerald H. K., Dunne A., Nat. Rev. Immunol., 2021, 21: 411-425
[11] Che J., Yang J., Zhao B., Shang P., Eur. J. Pharmacol., 2021, 906: 174219
[12] Wang Y., Gao L., Chen J., Li Q., Huo L., Wang Y., Wang H., Du J., Front. Pharmacol., 2021, 12: 757161
[13] Crocetti E., Bergamaschi W., Russo A. G., Eur. J. Gastroenterol. Hepatol., 2021, 33: e383-e389
 Abstract
Abstract
 Abstract
Abstract
 Abstract
Abstract
 Abstract
Abstract
 Coffee break
Coffee break Abstract
Abstract
 Abstract
Abstract
 Coffee break
Coffee break Abstract
Abstract
 Coffee break
Coffee break Abstract
Abstract
 Abstract
Abstract



 Arnaud Grandeury
Arnaud Grandeury